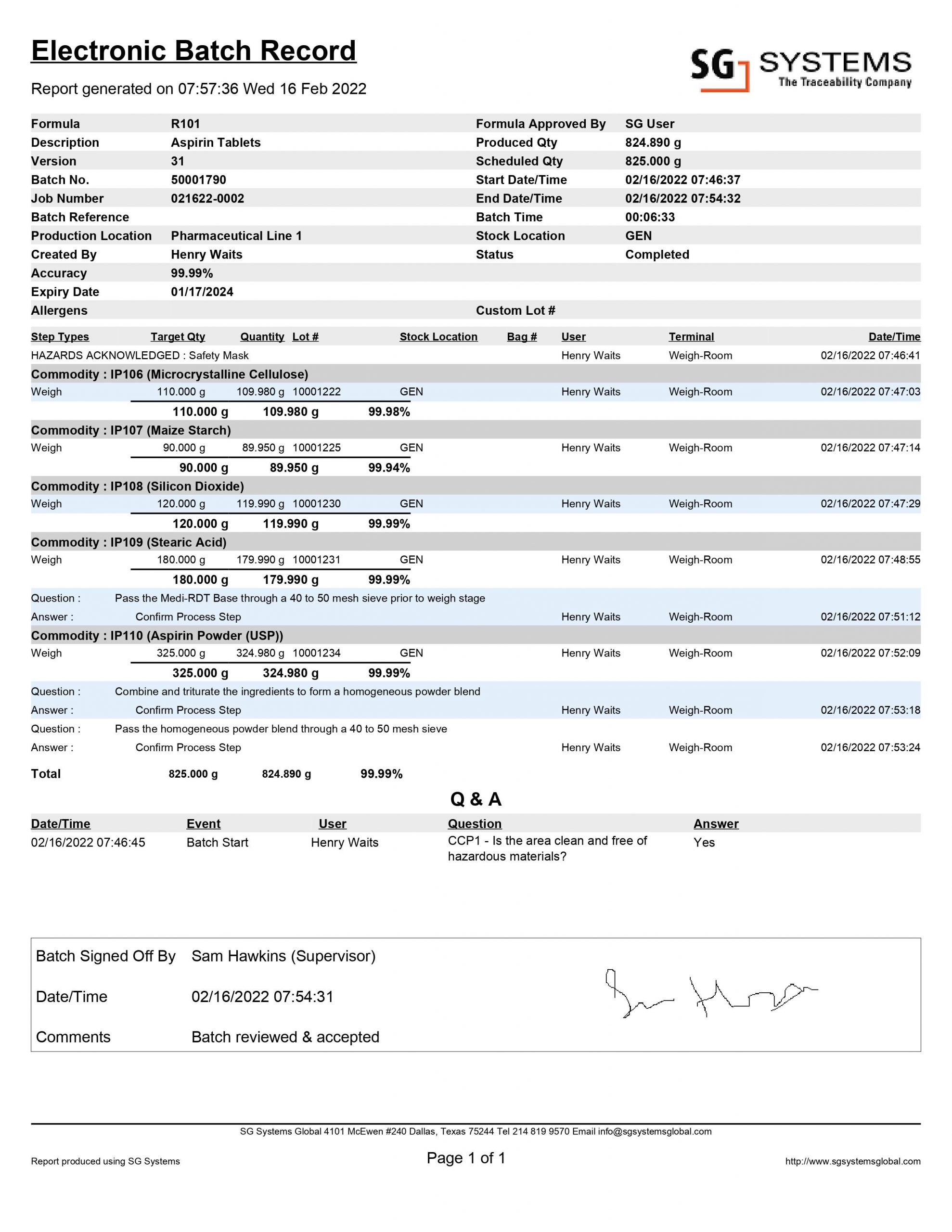
Batch Record MBR-046 Supersedes: New Version: 1.0 Title: Fexo A – Fexofenadine HCl tablets 60mg (F-7046) Effective: Page 1 of

ASEAN-TMHS-GMP-Training-Chapter-5-Annex-5-Sample-Batch-Manufacturing-Record.doc - COMPANY NAME Department : Production BATCH MANUFACTURING RECORD Title | Course Hero
Corso di Laurea Magistrale in Chimica e Tecnologia Farmaceutiche – E25 Fabbricazione Industriale dei Medicinali – 4 CFU

ASEAN TMHS GMP Training Chapter 5 Annex 5 Sample Batch Manufacturing Record | PDF | Tablet (Pharmacy) | Quality Assurance






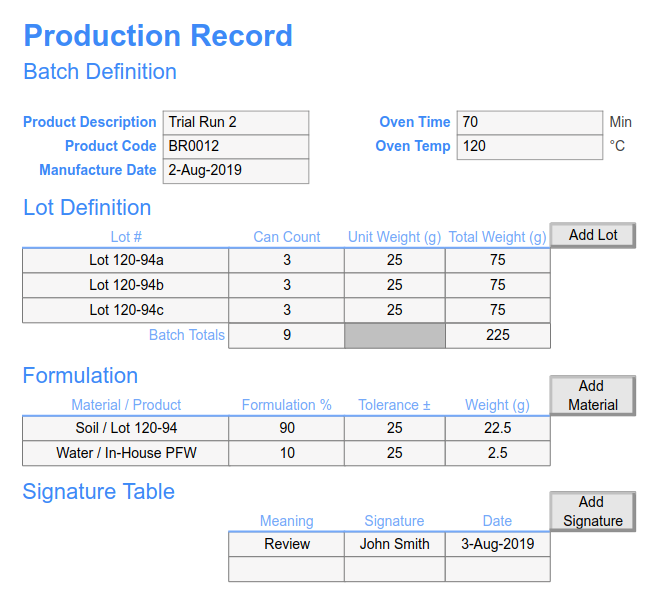
-en.jpg?Status=Master&sfvrsn=ce9a7fcc_0)